| → | CHOP INTEND |
| → | HINE |
| → | ULM、RULM |
| → | 6MWT |
| → | CMAP、MUNE |
Hammersmith運動功能評分量表-增訂版 (HFMSE) 已經用於多項臨床試驗,用以評估晚期發病型 (II型和III型) 脊髓性肌肉萎縮症病患的運動功能。 |
|
HFMSE量表包含主要動作功能評估量表(GMFM)中與躺臥/滾動、爬行、爬行/跪姿、站立、行走/跑步/跳躍相關的13個臨床項目4,14,15:
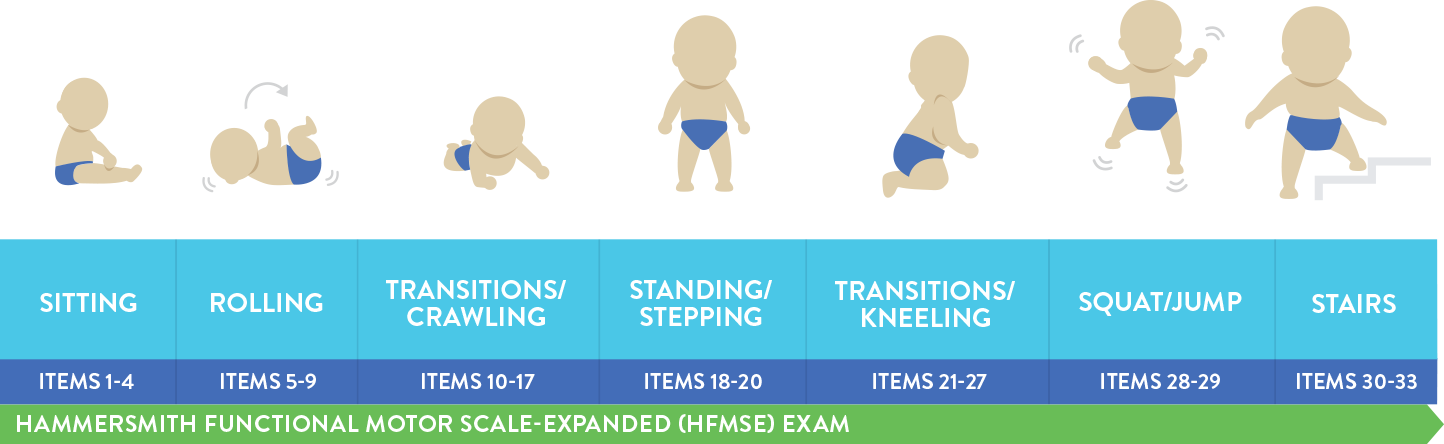
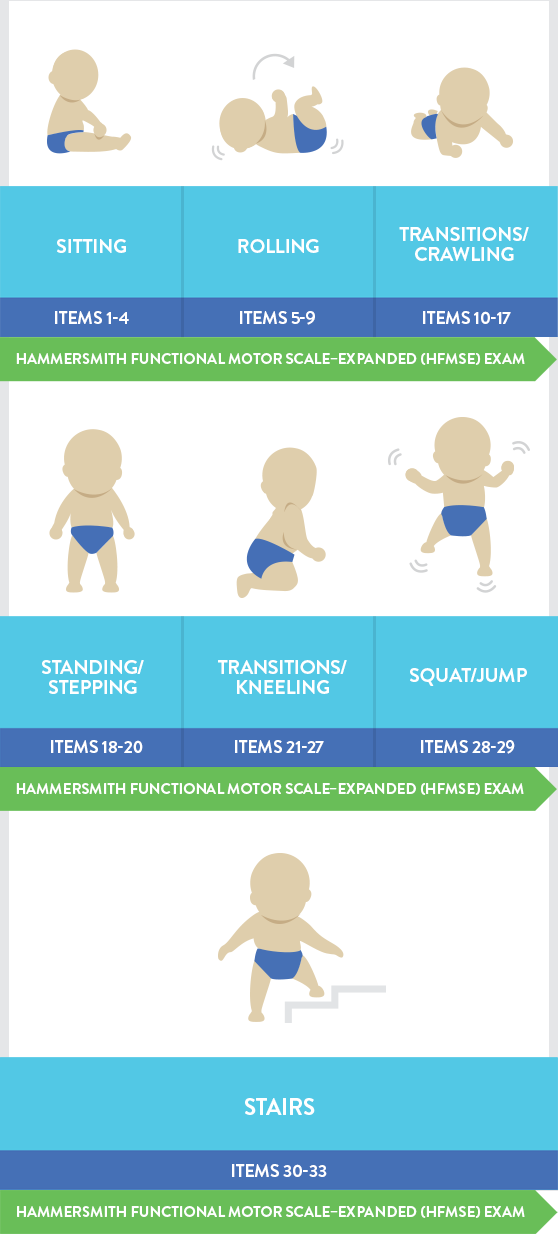
上圖中描繪的是大於或等於2歲的孩童。
|
|
晚期發作型SMA患者(II型和III型)的HFMSE評分可能會逐漸下降。4 |
|
|
一項SMA的自然病史研究報告顯示,晚發型SMA病患的HFMSE評分12個月內下降0.56分(平均值)。4 然而在另一項研究中,晚發型SMA病患(n=79)的運動功能以非線性方式下降。HFMSE評分在36個月內的平均變化為-1.71(P = 0.01)。 研究期間中16:
|
REFERENCES
1. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. 2. Montes J, Gordon AM, Pandya S, De Vivo DC, Kaufmann P. Clinical outcome measures in spinal muscular atrophy. J Child Neurol. 2009;24(8):968-978. 3. Darras BT, Royden Jones H Jr, Ryan MM, De Vivo DC, eds. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician’s Approach. 2nd ed. London, UK: Elsevier; 2015. 4. Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord. 2016;26(2):123-131. 5. Kolb SJ, Coffey CS, Yankey JW, et al; the NeuroNEXT Clinical Trial Network and on behalf of the NN101 SMA Biomarker Investigators. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol. 2016;3(2):132-145. 6. Data on file. Biogen Inc, Cambridge, MA. 7. Haataja L, Mercuri E, Regev R. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2 pt 1):153-161. 8. Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. 2016;58(3):240-245. 9. De Sanctis R, Coratti G, Pasternak A, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26(11):754-759. 10. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155-161. 11. Glanzman AM, McDermott MP, Montes J. Validation of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. 2011;23(4):322-326. 12. Spinal Muscular Atrophy Clinical Research Center. CHOP INTEND for SMA Type I score sheet. http://columbiasma.org/links.html. Updated March 14, 2013. Accessed April 26, 2016. 13. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016; 388(10063):3017-3026. 14. Glanzman AM, O’Hagen JM, McDermott MP, et al; the Pediatric Neuromuscular Clinical Research Network for Spinal Muscular Atrophy (PNCR), and the Muscle Study Group (MSG). Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26(12):1499-1507. 15. The Pediatric Neuromuscular Clinical Research Network for SMA. Expanded Hammersmith Functional Motor Scale for SMA (HFMSE). http://columbiasma.org/links.html. March 7, 2009. Accessed April 25, 2016. 16. Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79(18):1889-1897. 17. Sivo S, Mazzone E, De Sanctis, et al. Upper limb module in non-ambulant patients with spinal muscular atrophy: 12 month changes. Neuromuscul Disord. 2015;25(3):212-215. 18. Mazzone E, Bianco F, Martinelli D, et al. Assessing upper limb function in nonambulant SMA patients: development of a new module. Neuromuscul Disord. 2011;21(6):406-412. 19. Mazzone ES, Mayhew A, Montes J, et al. Revised Upper Limb Module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2016. doi:10.1002/mus.25430. 20. Montes J, McDermott MP, Martens WB, et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833-838. 21. Mazzone E, Bianco F, Main M, et al. Six minute walk test in type III spinal muscular atrophy: a 12 month longitudinal study. Neuromuscul Disord. 2013;23(8):624-628. 22. Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704-712. 23. Arnold WD, Sheth KA, Wier CG, et al. Electrophysiological motor unit number estimation (MUNE) measuring compound muscle action potential (CMAP) in mouse hindlimb muscles. J Vis Exp. 2015;103:1-8. 24. Bromberg MB, Swoboda KJ. Motor unit number estimation in infants and children with spinal muscular atrophy. Muscle Nerve. 2002;25(3):445-447. 25. Monti RJ, Roy RR, Edgerton VR. Role of motor unit structure in defining function. Muscle Nerve. 2001;1;24(7):848-866. 26. Finkel RS. Electrophysiological and motor function scale association in a pre-symptomatic infant with spinal muscular atrophy type I. Neuromuscul Disord. 2013;23(2):112-115.
SMA患者的基本活動能力與病情進展如何決定日常活動與生活方式?
了解更多