測量:運動功能
罹患SMA的嬰兒與兒童
(年齡在4個月至4歲以上)
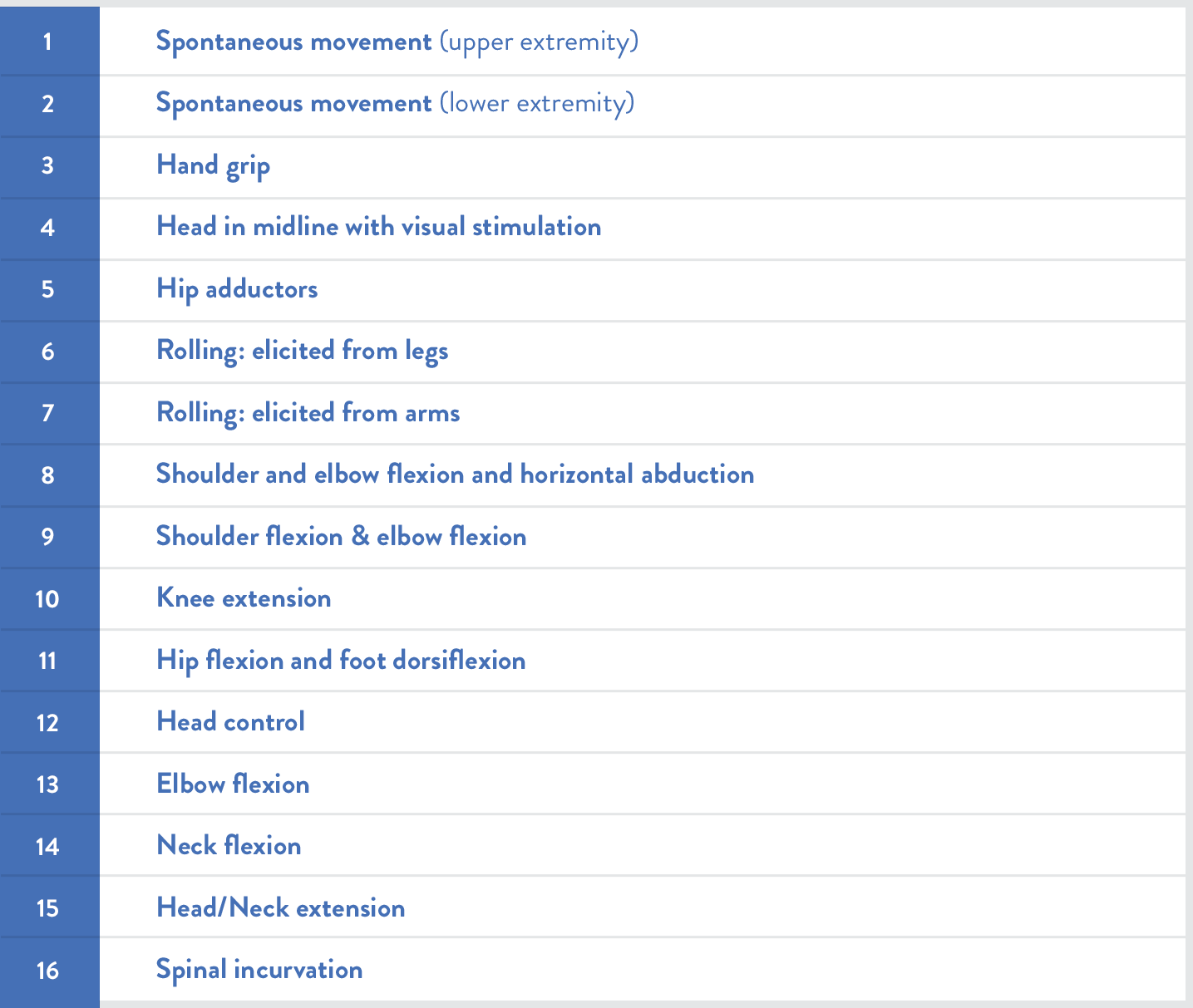
費城兒童醫院嬰兒神經肌肉疾病測試(CHOP INTEND)可用於評估SMA嬰兒的運動功能10,11: |
|
|
|

|
SMA嬰兒的CHOP INTEND分數可能明顯低於非SMA嬰兒
Kolb等人對經基因確診的SMA嬰兒進行了前瞻性長期追蹤的疾病自然史研究,對照SMA嬰兒與健康嬰兒的CHOP INTEND評量分數。SMA症狀發病年齡從小於1個月至4〜5個月不等。5
病患種類 |
CHOP INTEND分數(平均值) |
納入研究時年齡(平均值) |
SMA發病年齡 |
病患種類健康嬰兒(n=14) |
CHOP INTEND分數(平均值)50.1分 |
納入研究時年齡(平均值)3.3個月 |
SMA發病年齡
|
病患種類罹患 SMA†的嬰兒 的嬰兒(n=16)(n=16) |
CHOP INTEND分數(平均值)20.2分 |
納入研究時年齡(平均值)3.7個月 |
SMA發病年齡<1 個月 (6/16)
|
對於已出現症狀且只有兩個SMN2基因拷貝數的SMA I型患者而言,
CHOP INTEND評量分數大於40是很罕見的。13
REFERENCES
1. Finkel RS, McDermott MP, Kaufmann P, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810-817. 2. Montes J, Gordon AM, Pandya S, De Vivo DC, Kaufmann P. Clinical outcome measures in spinal muscular atrophy. J Child Neurol. 2009;24(8):968-978. 3. Darras BT, Royden Jones H Jr, Ryan MM, De Vivo DC, eds. Neuromuscular Disorders of Infancy, Childhood, and Adolescence: A Clinician’s Approach. 2nd ed. London, UK: Elsevier; 2015. 4. Mercuri E, Finkel R, Montes J, et al. Patterns of disease progression in type 2 and 3 SMA: implications for clinical trials. Neuromuscul Disord. 2016;26(2):123-131. 5. Kolb SJ, Coffey CS, Yankey JW, et al; the NeuroNEXT Clinical Trial Network and on behalf of the NN101 SMA Biomarker Investigators. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol. 2016;3(2):132-145. 6. Data on file. Biogen Inc, Cambridge, MA. 7. Haataja L, Mercuri E, Regev R. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2 pt 1):153-161. 8. Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. 2016;58(3):240-245. 9. De Sanctis R, Coratti G, Pasternak A, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26(11):754-759. 10. Glanzman AM, Mazzone E, Main M, et al. The Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): test development and reliability. Neuromuscul Disord. 2010;20(3):155-161. 11. Glanzman AM, McDermott MP, Montes J. Validation of the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND). Pediatr Phys Ther. 2011;23(4):322-326. 12. Spinal Muscular Atrophy Clinical Research Center. CHOP INTEND for SMA Type I score sheet. http://columbiasma.org/links.html. Updated March 14, 2013. Accessed April 26, 2016. 13. Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016; 388(10063):3017-3026. 14. Glanzman AM, O’Hagen JM, McDermott MP, et al; the Pediatric Neuromuscular Clinical Research Network for Spinal Muscular Atrophy (PNCR), and the Muscle Study Group (MSG). Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26(12):1499-1507. 15. The Pediatric Neuromuscular Clinical Research Network for SMA. Expanded Hammersmith Functional Motor Scale for SMA (HFMSE). http://columbiasma.org/links.html. March 7, 2009. Accessed April 25, 2016. 16. Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology. 2012;79(18):1889-1897. 17. Sivo S, Mazzone E, De Sanctis, et al. Upper limb module in non-ambulant patients with spinal muscular atrophy: 12 month changes. Neuromuscul Disord. 2015;25(3):212-215. 18. Mazzone E, Bianco F, Martinelli D, et al. Assessing upper limb function in nonambulant SMA patients: development of a new module. Neuromuscul Disord. 2011;21(6):406-412. 19. Mazzone ES, Mayhew A, Montes J, et al. Revised Upper Limb Module for spinal muscular atrophy: development of a new module. Muscle Nerve. 2016. doi:10.1002/mus.25430. 20. Montes J, McDermott MP, Martens WB, et al. Six-minute walk test demonstrates motor fatigue in spinal muscular atrophy. Neurology. 2010;74(10):833-838. 21. Mazzone E, Bianco F, Main M, et al. Six minute walk test in type III spinal muscular atrophy: a 12 month longitudinal study. Neuromuscul Disord. 2013;23(8):624-628. 22. Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57(5):704-712. 23. Arnold WD, Sheth KA, Wier CG, et al. Electrophysiological motor unit number estimation (MUNE) measuring compound muscle action potential (CMAP) in mouse hindlimb muscles. J Vis Exp. 2015;103:1-8. 24. Bromberg MB, Swoboda KJ. Motor unit number estimation in infants and children with spinal muscular atrophy. Muscle Nerve. 2002;25(3):445-447. 25. Monti RJ, Roy RR, Edgerton VR. Role of motor unit structure in defining function. Muscle Nerve. 2001;1;24(7):848-866. 26. Finkel RS. Electrophysiological and motor function scale association in a pre-symptomatic infant with spinal muscular atrophy type I. Neuromuscul Disord. 2013;23(2):112-115.
SMA患者的基本活動能力與病情進展如何決定日常活動與生活方式?
了解更多